Blueberry Therapeutics Meets Primary and Secondary Endpoints in Phase 2b Trial in Onychomycosis
Primary endpoint (negative culture and/or clear nail growth) met with 83.3% response rate Secondary endpoints met at Week 52 after 9-months off treatment Blueberry Therapeutics Limited (“Blueberry” or “the Company”), a pharmaceutical company focused on developing innovative, topical nanomedicines [...]
A warm welcome to Megan!
We’d like to extend a warm welcome to our new Laboratory Technician, Megan Dawson. Megan joins us with a Masters in Medical Microbiology from the University of Manchester, and after gaining lots of relevant skills in an associate practitioner [...]
Phase 2b Trial in Fungal Nail Infection Completed
Alderley Park, 04 July 2023 - Blueberry Therapeutics is pleased to announce the completion of our European Phase 2b trial of BB2603 (BBTAF202). This trial is assessing the efficacy and safety of different doses of BB2603, a new topical anti-fungal [...]
Update on Phase 2b Trial in Fungal Nail Infection
Alderley Park, 17 February 2023 - Blueberry Therapeutics is pleased to announce further progress on our Phase 2b European trial of BB2603 (BBTAF202). We previously announced the completion of recruitment, and we now provide an update that the final [...]
Blueberry Therapeutics Announce Nomination of BB1511 for Clinical Development for the Treatment of Atopic Dermatitis (Eczema)
Alderley Park, 22 November 2022 - Blueberry Therapeutics are pleased to announce that their novel cream formulation for the treatment of atopic dermatitis (BB1511) has been nominated for clinical development. This nomination decision was unanimous following review of pharmaceutical [...]
A new Analytical Scientist joins the Preclinical team
Blueberry extends a warm welcome to Sinead Sunner, who joins our preclinical team as an Analytical Scientist. Since graduating with an MSc with Honours in Medicinal and Biological Chemistry from the University of Nottingham, Sinead has worked at LCG [...]
Blueberry Therapeutics Announce Recruitment Complete to Phase 2b Trial
Alderley Park, 29 July 2022 - Blueberry Therapeutics are pleased to announce that recruitment into our Phase 2b European trial of BB2603 for fungal nail infection is now complete. This trial (BBTAF202) assesses the efficacy and safety of different [...]
Blueberry Therapeutics Provides Regulatory Update on Phase 2b Trial
Alderley Park, 24 May 2022 - Blueberry Therapeutics are pleased to report significant progress in our Phase 2b European trial of BB2603 in onychomycosis (BBTAF202). The COVID-19 pandemic led to operational challenges in all 3 participating countries (Germany, Poland [...]
Blueberry Therapeutics provides R&D update and reports progress across dermatology portfolio
Phase 2 trial with BB2603 in onychomycosis: continues, with initial readout expected in Q1 2023 Phase 3 trial with BB1202 for tinea pedis: ready to enter late-stage clinical development with Blueberry seeking advice from regulatory agency to finalise trial [...]
The Blueberry Team complete 546 km challenge to raise money for In2Science!
From the 19-25th April, Blueberry employees took on a challenge to collectively reach a target distance of 200 km in one week to raise money for the charity In2Science. Our active bunch answered this call by walking, running, swimming, [...]
Blueberry Therapeutics announces extension of its Series B round with investment from Medical Incubator Japan and US Dermatology Syndicate
Blueberry Therapeutics Limited would like to announce that it has completed a £3.6m extension to the previously successful Series B Fundraising of £10.8m. This investment is from a Japanese investor, Medical Incubator Japan (MIJ) and a private syndicate of [...]
Blueberry expansion at Alderley Park
Blueberry expansion at Alderley Park. The Blueberry team are delighted to be moving into a larger, more spacious office area on the Alderley Park site, with views over the Cheshire plains. The move will bring the whole team together [...]
Senior Analytical Scientist completes LC-MS training
Blueberry’s Senior Analytical Scientist Dr Heather Davies-Strickleton recently attended LC-MS Interpretation Level 1 training. Dr Davies-Strickleton said “This is an exciting opportunity for the Blueberry team to extract as much data as we can from the samples we analyse on [...]
CSO David Cook presenting at Alderley Park Science Seminar series
Blueberry's CSO, Dr David Cook has been invited to present at the Alderley Park Science Seminar series with his talk "Understanding what makes a good drug discovery project: can we ever really predict success?" on Tuesday 21st July 2020 12.00-13.00. [...]
Blueberry featured on Disruption Hub article for COVID-19 efforts
We are delighted to be featured on this month's Disruption Hub article "Businesses Rise to the Covid-19 Challenge". You can read the full article on our efforts here: https://disruptionhub.com/businesses-rise-to-the-covid-19-challenge
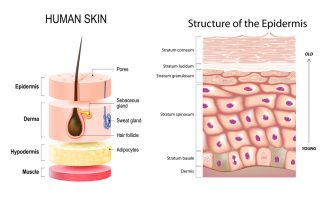
Getting under your skin: how a fungus takes hold
Written by Heather Davies-Strickleton, Senior Analytical Scientist We are not alone in this world. Although we can’t see them, we share our surroundings with billions of tiny microscopic organisms (microbes). Like us, they’re looking for a cosy place to [...]
Blueberry publishes latest peer-reviewed scientific paper
Today, Blueberry Therapeutics published our latest peer-reviewed scientific paper. The paper entitled “Assessment of the nail penetration of antifungal agents, with different physico-chemical properties” was published in PLoS One (https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0229414) and describes our research into antifungal drugs and their ability [...]
Blueberry Therapeutics extends a warm welcome to Clinical Science Lead
Blueberry Therapeutics are delighted to welcome Kerry Nield as Clinical Science Lead, with responsibility for clinical trial design and pipeline implementation including clinical scientific expertise, clinical strategy and product development planning. Kerry has over 17 years’ experience in pharmaceutical and [...]
Blueberry strengthens its analytical capabilities in the lab
Today, Blueberry announces a significant growth in its analytical capabilities with the investment in a new triple quadrupole mass spectrometry - the Agilent Ultivo LC/MS system. This instrument offers the ability to perform high quality quantitative analyses in support of all [...]
A new Clinical Project Manager joins the Blueberry team
Blueberry Therapeutics are delighted to welcome Sally Nguyen as a Clinical Project Manager, responsible for overseeing and managing the running of clinical trials from start up to completion/closeout. Sally possesses 12 years experience within Clinical Research, mainly [...]
Blueberry Therapeutics receives clearance from the FDA to proceed with the clinical investigation of BB2603
Blueberry Therapeutics has received an investigational new drug (IND) clearance from the Food and Drug Administration (FDA) to proceed with the clinical development of BB2603 for the treatment of onychomycosis. The IND is a crucial milestone in the continued [...]
Blueberry Therapeutics Appoints Dr. Adrian Howd to Board of Directors
Drug discovery and development company Blueberry Therapeutics announced the appointment of Dr. Adrian Howd to its Board as an independent Non-Executive Director, strengthening its strategic, financial and therapeutic expertise. Dr. Howd has over 20 years’ experience in the life science sector. He has held various private and public company Executive and Board roles in the UK, [...]
Blueberry Therapeutics completes BB2603 Investigator meeting
The Blueberry Therapeutics project team along with CEO John Ridden, and their partner Iqvia Biotech, held an Investigator meeting in Vienna, Austria on November 8th 2019. The meeting was attended by over 50 site staff from both private and [...]
Blueberry Therapeutics extends a warm welcome to Emma Leigh
Blueberry Therapeutics are delighted to welcome Emma Leigh as Medical Writing Lead, responsible for oversight of medical writing across nonclinical, clinical and regulatory deliverables. Prior to joining Blueberry, Emma has 18 years’ experience as a regulatory/medical writer within pharmaceutical companies [...]
John Ridden invited to showcase Blueberry’s success at BioCap
Blueberry CEO John Ridden has been invited to speak at BioCap this October at Alderley Park. John is pleased to have been invited to showcase the company’s success over the last 6 years. The 1-day conference allows for significant networking [...]